Digital Solutions for the Health Products Industry
I. Core Industry Pain Points
Regulatory Compliance Pressure: The National Medical Products Administration requires the establishment of a drug traceability system for full-process traceability.
Data Silo Phenomenon: The industry's informatization level lags, full-link data is incomplete, and efficiency is low.
Channel Management Challenges: Distribution channels are complex, and diversion of goods is frequent, disrupting market order.
Counterfeiting & Substandard Product Risks: Counterfeit drugs harm consumer health and damage enterprise brands and interests.
II. QS Digital Platform Solution
Centered on "one item, one code" technology, we build a full-process digital management system:
Drug Quality and Safety Traceability:
Covers data collection across the entire chain of raw materials, production, warehousing, and sales, connecting codes with data to establish a one-stop traceability system.
Consumers can scan codes to query full-process information from raw materials to finished products, enhancing trust.
Anti-Diversion Management System:
Establishes product associations through coding, automatically collecting scan data from distributors, retail stores, and consumers.
The system compares against distribution ranges, intelligently alerting for diversion and providing real-time feedback to headquarters.
Digital Channel Control:
Achieves full-process digital management of warehousing, distributors, and retail stores.
Through real-time inventory and sales data, it helps enterprises precisely plan production, supply, and sales.
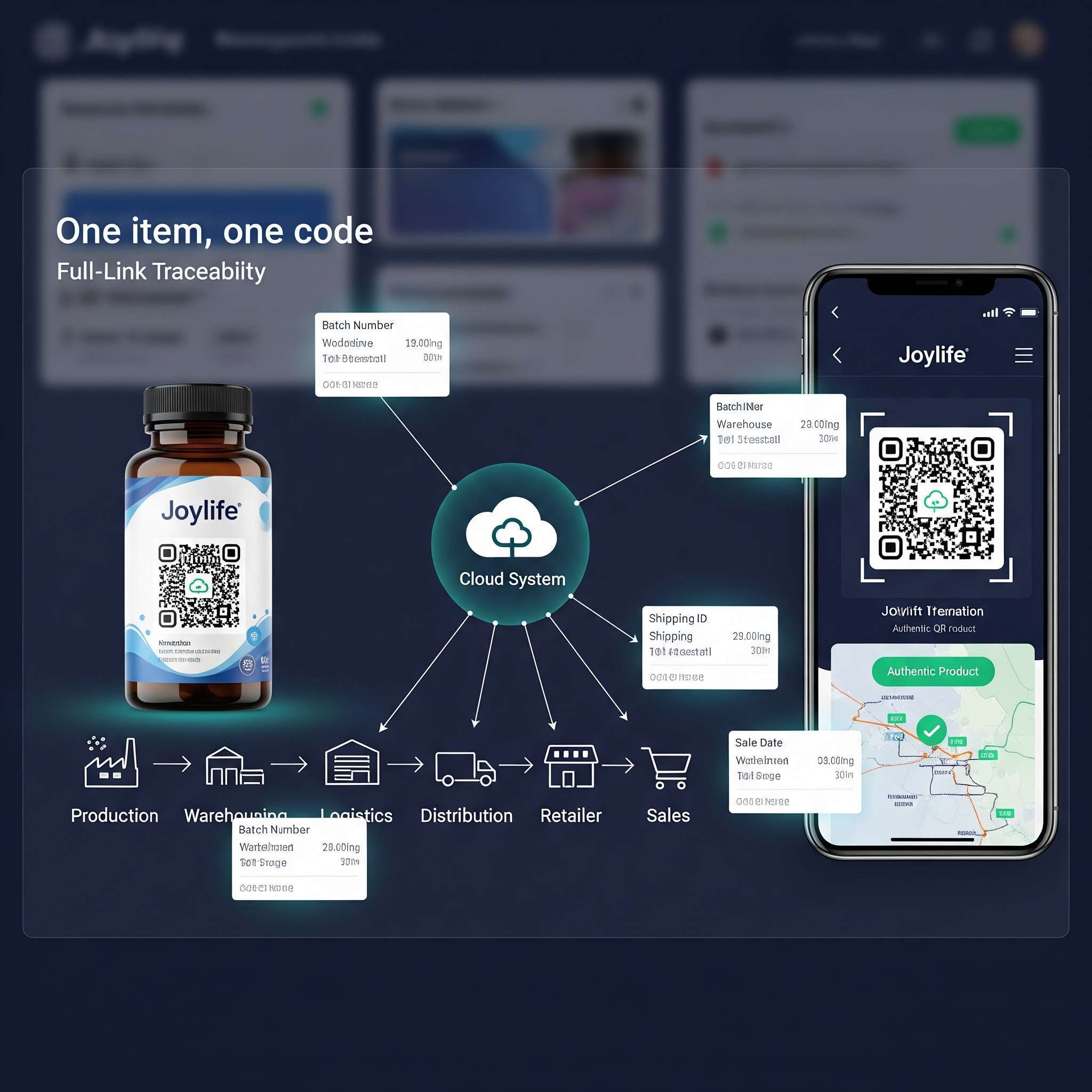
III. Classic Case Study: Joylife
Company Background:
Founded in 2010, with strategic partnerships with top scientific research institutions and authoritative organizations like Tianya Community and Changxing Pharmaceutical.
Has obtained GMP certification and received honors such as "Most Trusted Quality Brand by Consumers" and "Top Ten Chinese Health Food Brands."
Solution & Achievements:
(1) Brand Anti-Counterfeiting System:
Used "one item, one code" technology to give each product a unique digital label, combined with online anti-counterfeiting queries to ensure the circulation of genuine products.
(2) Full-Link Anti-Diversion:
Through automatic cap labeling and in-box scanning, it established a bottle-box-pallet association, achieving product data tracking and digital anti-diversion management.
(3) Visualized Full Lifecycle Traceability:
Solution Value:
Compliance: Meets national traceability system regulatory requirements and reduces legal risks.
Efficiency Improvement: Breaks down data silos, achieves full-link informatization, and optimizes production, supply, and sales decisions.
Market Purification: Curbs diversion and counterfeiting, maintaining channel order and consumer rights.
Brand Value-add: Enhances consumer trust and brand competitiveness through traceability and anti-counterfeiting.
This solution, empowered by technology, helps health product companies build a trustworthy, efficient, and transparent digital ecosystem to address industry challenges and achieve sustainable development.
